

SI unit for molar mass is kg/mol and other units that could be used to symbolise molar mass also includes, g/mol. Molar Mass (M)=GramsMoles kg/mol (or) g/mol A mole is unit of measurement that aims to compre particles of any given chemical compound to its mass. The unit used to measure is grams per mole. Molar mass of a chemical element is the sum of the total mass in grams of all the atoms that make up a mole of a particular molecule. The most dominants elements in dry air include oxygen, carbon dioxide, nitrogen, and argon and combining these elements along with some trace particulates of other chemicals, the molecular mass of air can be calucuated to be approximately 28.9647 g / mol (TET, n.d.). The air around us is a mixture of several components and gases, and each of these brings in its own characteristics to the overall health of the air around us. What is the molecular mass or weight of air? The number of grams of KClO3 will be 306.The below article talks about the molecular mass of air, the components that contribute towards this, along with some frequently asked questions about the molecular mass of air and other factors that affect the molecular mass of air.


Divide the given mass (25.0 g) by the molar mass (158.032 g/mol) to get the moles.
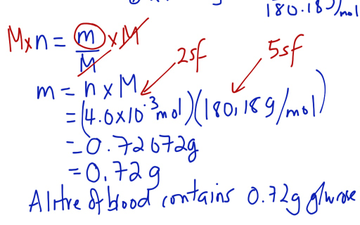
The molar mass of KMnO4 is 158.032 g/mol. Problem: Convert 25.0 grams of KMnO4 to moles.įind out the molar mass of the substance (hint: you can use Molar mass of the substance alone to calculate molar mass). Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities (i.e., pure numbers), whereas molar masses have units (in this case, grams/mole).Īnd this is where our grams to moles/moles to grams calculator shines, thanks to our other calculator - Molar mass of the substance, which calculates molar mass for a substance given the formula.Īll you need to do is correctly enter your formula, choose whether you want a conversion from grams to moles or a conversion from moles to grams, and, in case of g to mol, enter the mass, or, in case of mol to g, enter the moles. The molar mass of a compound is given by the sum of the standard atomic weight (namely, the standard relative atomic mass) of the atoms which form the compound multiplied by the molar mass constant. The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant, 1 × 10−3 kg/mol = 1 g/mol. The molar mass is a physical property defined as the mass of a given substance (chemical element or chemical compound) divided by the amount of substance. You need to multiply the molar mass of the substance by the number of moles:Īs you can see, the most difficult task here is finding out the molar mass of the substance. You need to divide the mass of the substance by the molar mass of the substance:
NO MOLAR MASS HOW TO
molar mass of the substance in grams/mole How to convert grams to moles? There is a simple relation between grams and moles:


 0 kommentar(er)
0 kommentar(er)
